Particle Dispersion and Energy-Storage Materials Lab
微粒子分散與儲能材料實驗室
Porous Microspheres

A new type of designed copolymers has been synthesized for the formation of porous polymeric microspheres. The copolymers contain hydrophobic (styrene, methyl methacrylate, vinylbenzyl chloride, or vinylbenzyl ethyl ether) and hydrophilic (vinylbenzyl alcohol) repeating units. Since the specially designed copolymers have unique chemical and physical properties, the porous structure can be easily accomplished by a facile single-step process. The resulting porous microspheres exhibit good morphological quality, showing open pore structure with a pore size ranging from submicrometer to micrometer, by neither use of porogens nor the requirement of complicated multistep emulsifications. The discovery for the exceptional performance of pores in microspheres is exciting and groundbreaking. The chemical features of the proposed copolymers for the availability in the formation of porous architecture provide important insights into the design principle of high quality porous structures.

The zirconia crucible containing the black powder carbonized from the swelled PSV porous microspheres. Raman spectrum of porous carbon spheres prepared from swelled PSV microspheres (compared with commercial carbon black).
Compared to conventional dense materials, porous materials exhibit special features such as relatively low density, high surface area, light weight, sound and thermal insulation, and good permeating selectivity. These remarkable properties have made porous materials of great scientific and technological interest, enabling their use in a wide range of industrial applications and products, including efficient adsorbents for storage and controlled release, carriers for medicines and biomaterials, supports for conversion reactions, supercapacitors, batteries, solar power, and fuel cells. With an increased demand for new materials in surface-related applications, research into developing fabrication techniques for porous materials has increased. Among material types and architectures, polymeric porous spheres have been the highest developed, and they are also common precursors and templates for other materials like carbons, metals, and ceramics in the fabrication of porous structures.

Microcapsules

Microcapsules have attracted attention in the field of novel and advanced materials due to their potential applications in hightech industries. The advantage of encapsulating specific materials in the core of a microcapsule is that the core materials can be quarantined to function only at the right time, i.e. they will remain stable inside the microcapsule until they are triggered. Due to wide variety of species available for the core materials, microcapsules have the potential to be employed in a wide industrial products, for instance, food and cosmetic additives, drug delivering carriers for bio-material and medicine fields, self-healing additives for microstructural and functional restorations and so on. Among these applications, the self-healing function of microcapsules has attracted the most interest in recent decades. The research team of Scott R. White et al. was the first to reveal the potential for utilizing microcapsules as self-healing materials. From their report in 2001, they successfully embedded the microcapsules of poly(urea–formaldehyde) (PUF) in resin which was cast on the surface of a certain substrate that needs to be protected or be able to restore itself as needed. Based on the healing mechanism, not only the structural fracture but other physical properties such as anti-corrosion or electrical conductivity can also be spontaneously restored.




Cross-sectional SEM image of a broken microcapsule embeddeding resin, shell thickness about 50-100 nm

SEM images of (a)microcapsules (b)microcapsules with Ag coated (c)Aqueous suspensions of microcapsules (left) and Ag-coated microcapsules.

Since the shell of most microcapsules is primarily polymeric which is mechanically soft and less compatible with lots of inorganic materials, the utilization of microcapsules is generally circuitous; the microcapsules are embedded in a polymeric film on the top of the target substrate that needs the self-healing function. This is especially true when the substrate is a metal- or ceramic-based material because of the very different surface tensions. This procedure makes the use of microcapsules complicated and limits their use in other applications. On the other hand, the triggering force may decay during transmission and only the microcapsules near the interface between the polymer and the target substrate have the opportunity to function, while those embedded far from the interface will become useless. To make the use of microcapsules more convenient and more efficient in the healing process, wastage of microcapsules should be reduced and they should be buried directly in the target substrate.

(a) Variation in current before and after being damaged for three circuits with and without embedded 20vol% of PUF-C20 and Ag@mPUF-C20 microcapsules under a consstant applied voltage of 1 V. Schematic mechanism for restoration: (b1) direct embedding of microcapsules (green) in the Ag-based circuit matrix (gray) on a glass substrate (light blue); (b2) healing material in the core after damaged; (b3) melted healing material released; (b4) damaged recovered from both fillings of the Ag particles rearranged from the matrix and the re-solidified healing material. (c) Cross-sectional SEM image of recovered zone near the interface between the Ag matrix and glass for the microcapsules embedded circuit.

(a)Diagram of cracks may not be completely recovered when microcapsules are poorly distributed. (b) This diagram shows the high probability for cracks being restored when microcapsules are well-dispersed.
ref. RSC Adv., 5, 104145-104148, 2015.
Li-ion batteries

Ever since lithium iron phosphate (LiFePO4) was reported as a potential cathode-active material for a lithium-ion battery by Goodenough and his coworkers in 1997, it has attracted widespread attention and been extensively studied during the past decade. The advantages of the olivine-structured LiFePO4 include a large theoretical capacity, good lifecycle performance, and safety. The excellent structural stability of LiFePO4, which results from strong Fe-P-O bonds, also greatly increases its thermal stability at high temperatures in its fully charged state. In addition, the low cost and toxicity of LiFePO4 owing to its environmentally compatible constituents make it a promising cathode active material for large batteries.

Li-ion battery structure
ref: J. Mater. Chem. A, 2015, 3, 2454-2484

hybrid circuit co-fired ceramics
ref:wiki

nano-LiFePO4
LiFePO4 has some disadvantages, such as poor electrical conductivity (~10-9 cm-1) and the diffusion of lithium ions (Li+) in LiFePO4. These issues result in losses in capacity and rate capability and thus hinder the commercial application of LiFePO4. The use of fine LiFePO4 particles has been proposed to improve Li+ diffusion. Furthermore, surface coating with a conductive material is a commonly used approach to enhance the electrical conductivity of LiFePO4. Among the various possible conductive coating materials, carbon is the most prevalent because of its high chemical stability. Commercially produced LiFePO4 powders are available with a varying amount of carbon content that typically ranges from 1 to 5 wt% because of the differences in techniques used for the synthesis of LiFePO4. For the fabrication of electrodes, electrode materials are typically mixed using either a water-based (aqueous) or solvent-based (non-aqueous) process. The aqueous process is gaining favor and has attracted significant interest because of its environmental consistency and cost considerations. However, the aqueous process has a drawback, i.e., the agglomeration of most oxides, including LiFePO4; until now, the only efficient approach to prevent powder agglomeration has been the addition of an appropriate dispersant to the system. Furthermore, several reports have noted that not all commercial LiFePO4 powders exhibit the same dispersity in aqueous slurries, i.e., notable differences in the dispersion properties of the aqueous slurries prepared with powders from different production lots made by the same supplier may be observed. This variety in the dispersity of LiFePO4 powder in water is an important issue that has caused great concern in LiFePO4-related industries. In addition, the indeterminate dispersity of the powders may cause end users to manipulate them imprecisely resulting in unsuitable electrode slurries. Typical commercially available LiFePO4 powders are obtainable as both dispersions and gels in water. Dispersible LiFePO4 (D-LFP) and gelled LiFePO4 (G-LFP) are two such LiFePO4 powders with the same physicochemical properties of crystallinity, a median particle size (d50) of 2.2 mm, and an approximate carbon content of 1.07-1.20 wt%; these powders were acquired from the same supplier. When we processed them in water by adding the same ingredients, different distinctive flow behaviors of the as-prepared aqueous slurries were observed. The aqueous slurry prepared from the D-LFP powder shows fluidity, whereas the slurry from G-LFP resembled a jelly-like gel. As the formation of powder gel will be detrimental to the electrode-manufacturing process, especially for the slurry-sieving and slurry-casting steps, understanding the cause for the deviation in the dispersity of powders is essential and a prerequisite.

ref. J. Power Sources, 310, 45-53, 2016.
Dispersion



Titania (TiO2) is an important and widely used material in industries ranging from traditional to highly technical because of its attractive and extensive physicochemical properties. TiO2 must be compatible with other materials for it to distribute homogeneously in composites and for it to be useful in various applications. Therefore, the dispersity of TiO2, which is determined by its surface properties, is an important issue that was examined in past decades. Commercial TiO2 nanopowders usually exhibit a variety of surface properties. For instance, they show acid–base properties that vary with the manufacturer and production process. Different manufacturers or manufacturing processes may use a variety of dopants for TiO2 to modify or improve its physicochemical properties, such as thermal stability and chemical activity. As a result, the surface chemistries of commercial TiO2 are frequently unclear, making it difficult to control its dispersion.
ref. J. Am. Ceram. Soc. in press, 2016.
Nano materials


nano-diamond
nano-diamond suspension

nano-diamond suspension (after being dispersed)

BaTiO3@SiO2 core-shell

nano-silver
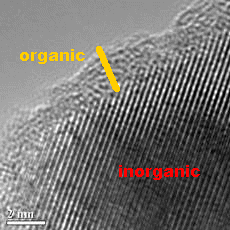
nano-BaTiO3 (after being dispersed)

nano-silver (after dispersion)
Composite materials





